Food And Drug Act Timelines
And g International Trade in Endangered Species Act 2008 Act 686. 301 as amended the drug is required to bear a label containing the legend Caution.

Food Drug And Cosmetic Act Lessons Blendspace
Timeline Event List Page Number Paper Orientation More Options Paper Size.

Food and drug act timelines. It was a federal law that provided federal inspection of meat products and forbade the manufacture sale or transportation of adulterated food products and poisonous patent medicines CITATION Pri15 l 1033. Pure Food and Drug Act 1906 required ingredients on packaging. D Poisons Act 1952.
The purpose of this act was to ensure that all. FAQ about 1906 Pure Food And Drug Act Timeline What was the Food and Drug Act of 1906. Timeline of food and drug legislation.
Congress enacts the Sherley Amendment. The Pure Food and Drug Act of 1906 is a great revolution in which the Congress passed a legislation to force companies to list all ingredients of the product they sell. Pure Food and Drug Act The first Pure Food and Drug Act was passed in 1906 to protect people from adulteration of food and from products identified as healthful without scientific support.
The Federal Meat Inspection Act prohibited the sale of adulterated or misbranded meat and meat products for food and ensured that meat and meat products were slaughtered and processed under sanitary conditions. The Pure Food and Drug Act prevented the manufacture sale or transportation of adulterated or misbranded foods drugs medicines and liquors. Pure Food And Drug Act Definition Us History.
It prohibits interstate commerce in misbranded and adulterated foods and drugs. C Dangerous Drugs Act 1952. The original Food and Drugs Act is passed by Congress on June 30 and signed by President Theodore Roosevelt.
Federal law restricts this drug to use by or on the order of a licensed veterinarian or any similar. The FDA is responsible for protecting the public health by ensuring the safety efficacy and security of human and veterinary drugs biological products and medical devices. 1906 Pure Food and Drug Act a.
The purpose was to protect the public against adulteration of food and from products identified as healthful without scientific support. This benefits citizens in the US. Anti Drug Abuse Act 1986 Combat Methamphetamine Epidemic Act.
The date of filing begins the 180-day period described in. The Pure Food and Drug Act also known as the Wiley Act was passed on June 30 1906. Pharmacopeia the first compendium of standard drugs for the United States.
Muckraking journalists had long reported on the appallingly unsanitary conditions of the countrys. A Under the Food Drug and Cosmetic Act 52 Stat. On this date the Pure Food and Drug Act of 1906 PL 59-384 passed in the US.
1906 The original Food and Drugs Act is passed by Congress on June 30 and signed by President Theodore Roosevelt. FDAAA 801 and the Final Rule. It prohibits interstate commerce.
But the two main regulatory food agencies-the Department of Agriculture and the Food and Drug Administration-have no ruling on mandatory dating of food packages leaving it up to the manufacturer and packager whether to advise consumers on the. B Control of Drugs and Cosmetics Regulations 1984. The first Pure Food and Drug Act was passed in 1906.
House of Representatives 240 to 17. The date of filing will be the date 60 days after the date FDA received the NDA. Federal law prohibits dispensing without prescription or Caution.
The events leading to the Pure Food and Drug Act of 1906. 1964 USA---Food Stamp Act SNAP. The original Pure Food and Drug Act was amended in 1912 1913 and 1923.
Image courtesy of the Library of Congress The Pure Food and Drug Act was a centerpiece of progressive reforms in the early 20th century. The statutory requirements have been in effect since September 27 2007 have been codified at section. San Francisco Ordinance 1875.
E Medicines Advertisement. Muckrakers timeline Timetoast timelines. And by ensuring the.
The Pure Food and Drug Act was passed forming the Food and Drug. The Great American Fraud and the Pure Food and Drugs Act of 1906 The Sulfanilamide Disaster and the Federal Food Drug and Cosmetic Act of 1938 Eleven physicians meet in Washington DC to establish the US. The combination of the Poison Squad and The Jungle prompted Congress to pass the Pure Food and Drugs Act in 1906.
F Wildlife Conservation Act 2010 Laws of Malaysia Act 716. Federal controls over the drug supply namely banning the importation of adulterated drugs started in 1848. The Federal Food Drug and Cosmetic Act FDCA applies to a biological product subject to regulation under this section except that a product for which a license has been approved under subsection a shall not be required to have an approved application under section 505 of such Act.
Public became widespread as. It prohibits interstate commerce in misbranded and adulterated foods and drugs. This act did not apply to people outside of the United States.
This page summarizes the clinical trial registration and results information submission requirements described in Section 801 of the Food and Drug Administration Amendments Act of 2007 PDF known as FDAAA 801. _ 7 42 USC. Most federal laws concerning the FDA are part of the Food Drug and Cosmetic Act first passed in 1938 and extensively amended since and are codified in Title 21 Chapter 9 of the United States Code.
1040 1938 21 USCA. By letting consumers know exactly what was in their food. A group of twelve volunteers nicknamed the poison squad agree to eat food laced with common.
A Sale of Drugs Act 1952. Pharmacopoeia and National Formulary as official standards for the strength quality and purity of drugs and for the tests to make such determinations. The 1906 law recognized the privately produced US.

Fda S Approach To The Gras Provision A History Of Processes Fda
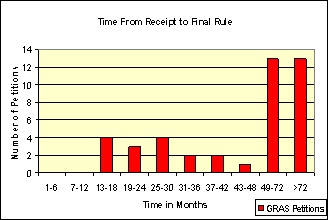
Fda S Approach To The Gras Provision A History Of Processes Fda

The Pure Food And Drug Act Us House Of Representatives History Art Archives

Part I The 1906 Food And Drugs Act And Its Enforcement Fda

Fda S Approach To The Gras Provision A History Of Processes Fda

History Brief Teddy S Food And Drug Regulation Youtube
The Long Struggle For The Law Fda

The Food And Drug Administration The Continued History Of Drug Advertising Weill Cornell Medicine Samuel J Wood Library
Food Drug And Cosmetic Act Lessons Blendspace

Timeline Of Events Defining The Pharmaceutical Industry In The Modern Download Scientific Diagram

The Food And Drug Administration The Continued History Of Drug Advertising Weill Cornell Medicine Samuel J Wood Library

The Food And Drug Administration The Continued History Of Drug Advertising Weill Cornell Medicine Samuel J Wood Library

The Food And Drug Administration The Continued History Of Drug Advertising Weill Cornell Medicine Samuel J Wood Library

Part I The 1906 Food And Drugs Act And Its Enforcement Fda

Part Ii 1938 Food Drug Cosmetic Act Fda
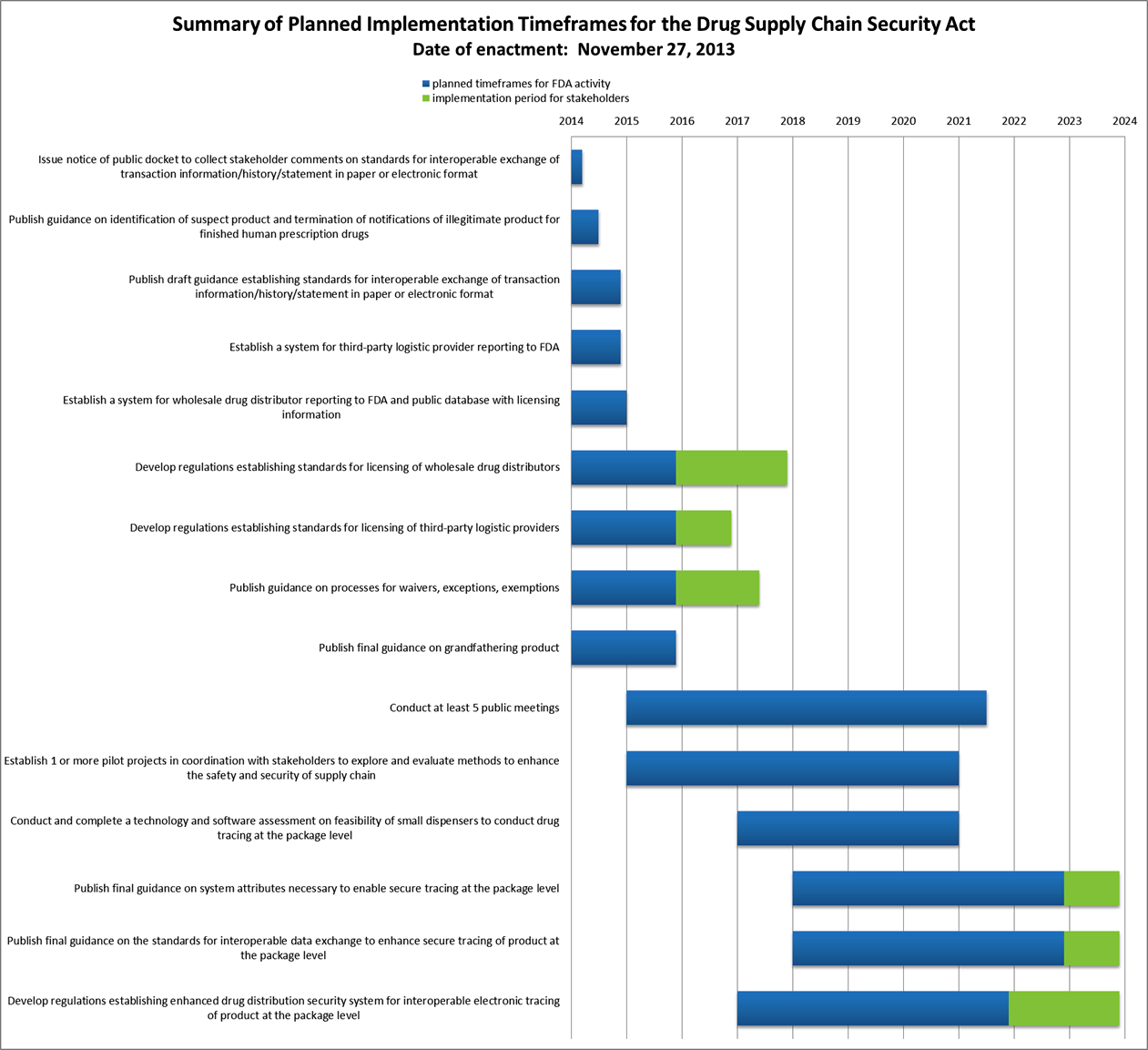
What You Need To Know About The Drug Supply Chain Security Act
Drug Supply Chain Security Act Dscsa Implementation Plan Fda

Pure Food And Drug Act Passed On This Day 1906 Gilder Lehrman Institute Of American History

Effects Of Food Regulation In The Progressive Era Docsteach

Post a Comment for "Food And Drug Act Timelines"