Food Drug And Cosmetic Act Enforcement
The conspiracy also involved aiding and abetting violations of the Food Drug and Cosmetic Act by facilitating the dispensing of its opioid products including OxyContin without a legitimate medical purpose and thus without lawful prescriptions. 006 Applicability of definitions in KRS 516010 to KRS 217207 217208 and 217209.

Part I The 1906 Food And Drugs Act And Its Enforcement Fda
1938 Federal Food Drug and Cosmetic Act 21 USC.

Food drug and cosmetic act enforcement. The objective of FDA regulatory programs is to assure compliance with the Federal Food Drug and Cosmetic Act the Act. In 1968 the Electronic Product Radiation Control. Food and Drug Administration to oversee the safety of food drugs medical devices and cosmetics.
The Federal Food Drug and Cosmetic Act was passed by Congress in 1938 in reaction to the growing public safety demands. This webinar will explore different views on how this decision might affect FCA investigations and litigation in the coming years including whether drug and device companies can expect more cases based on violations of provisions of the Federal Food Drug Cosmetic Act. Ing violations of food and drug regulations as evidenced by the presence of a number of criminal cases involving food and drug industry participants.
LPE As federal regulatory legislation goes the Food and Drugs Act of x9o6 has left in its wake an extensive administrative and judicial history. Specific enforcement activities include actions to. PART 1 -- GENERAL ENFORCEMENT REGULATIONS.
301 et seq as amended may be adopted as rules by referencing the federal regulations subject to the approval of the joint committee on agency rule. 321 et seq 1957 Poultry Products Inspection Act 21 USC. Senator from New York.
Copeland a three-term US. Federal Food Drug and Cosmetic Act. THE ENFORCEMENT PROVISIONS OF THE FOOD DRUG AND COSMETIC ACT FuDmiuc P.
A The provisions of regulations promulgated under the Federal Food Drug and Cosmetic Act with. The law also provided for federal oversight and enforcement of. Second the bill specifies that dietary supplements are food products under Ohio law.
This page provides an overview of FDAs import compliance and enforcement activities at. It therefore might be time to question the goals of the Food Drug and Cosmetic Act of 1938 enforcement policy and whether the use of strict criminal liability. The United States Federal Food Drug and Cosmetic Act is a set of laws passed by Congress in 1938 giving authority to the US.
Lastly the bill gives the Director of the Ohio Department of Agriculture the authority to carry out the bills enforcement and oversight provisions. 002 Individuals exempt from application of chapter. The primary goal of the Act is to protect the health and safety of the public by preventing deleterious adulterated or misbranded articles from entering interstate commerce.
KRS Chapter 217. FDA enforces the Federal Food Drug and Cosmetic Act FDC Act and other related acts. The abovementioned product was verified by FDA through postmarketing surveillance and shows no valid Certificate of Product Notification CPN as of 02 December 2021.
The Federal Food Drug and Cosmetic FDC Act 1988 provides that food is adulterated if it meets any one of the following criteria. The enforcement of the Federal Food Drug and Cosmetic Act Food Drug Cosmet Law Q. Includes enactments through the 2021 Special Session.
Enforcement of the Food Drug and Cosmetic Act. 139 rows FDA Debarment List Drug Product Applications Below is a public. Food Drug Cosmetic and Device Enforcement Amendments of 1991 - Amends the Federal Food Drug and Cosmetic Act FDCA to authorize any US.
This has made available much experience as to the effectiveness of the rather wide variety of enforcement. Under section 402 a 4 of the Act a food product is deemed. The following links go to the FDA web site.
Purdue also admitted it conspired to violate the federal Anti-Kickback Statute. Food Drug and Cosmetic Act. Phillips Associate Attorney Gibson Dunn Crutcher LLP.
A Under section 307 of the Federal Food Drug and Cosmetic Act the act a State may bring in its own name and within its own jurisdiction proceedings for the civil enforcement or to restrain violations of sections 401 403b 403c 403d 403e 403f 403g 403h 403i 403k 403q or 403r of the act if the food that is the subject of the proceedings is located in the. The KRS database was last updated on 11212021. District court to order the recall of a food drug device or cosmetic which is in violation of the FDCA if the violation involves fraud or presents a significant risk to human or animal health.
1040 1938 21 USCA. Portions of Titles 7 9 and 21 of the Code of Federal Regulations or the regulations adopted for the enforcement of the Federal Food Drug and Cosmetic Act 52 Stat. Federal Food Drug and Cosmetic Act Code of Federal Regulations Guidances Enforcement Actions Federal Food Drug and Cosmetic Act.
Select Legal Issues Congressional Research Service 2 for safety16 Congress enacted the FDC Act in 193817 acting pursuant to its constitutional authority to regulate interstate commerce18 The Acts primary purpose is to safeguard and. A principal author of this law was Royal S. The law established quality standards for food drugs medical devices and cosmetics manufactured and sold in the United States.
The Food and Drug Administration FDA warns the public from purchasing and using the unauthorized cosmetic product LIQUID HAND SANITIZER CLEAR. 005 Citation of KRS 217005 to 217215. First it adopts sections of the federal Food Drug and Cosmetic Act regarding dietary supplements.
The Federal Food Drug and Cosmetic Act of 1938 APA is a federal law passed in 1938. Subpart A - General Provisions. 451 et seq 2011 Food Safety and Modernization Act.
This part governs the practices and procedures applicable to regulatory enforcement actions initiated by the Food and Drug Administration pursuant to the Federal Food Drug and Cosmetic Act 21 USC. 301 et seq and other laws that it administersThis part also provides guidance for manufacturers and distributors to follow with respect to their voluntary removal or. The Federal Food Drug and Cosmetic Act and subsequent amending statutes are codified into Title 21 Chapter 9 of the United States Code.

Regulations Administrative Materials Food Drug And Cosmetic Law Research Guide Guides At Georgetown Law Library

Part Ii 1938 Food Drug Cosmetic Act Fda

Food Additive Reform Time To Repeal The Delaney Clause Food And Drug Law Institute Fdli
Part Ii 1938 Food Drug Cosmetic Act Fda

P Harmacy L Aws Pure Food And Drug Act Enacted To Stop Sale Of Inaccurately Labeled Drugs All Manufacturers Required To Put Truthful Info On Labels Ppt Download
Food And Drug Administration Selected Funding And Policy Issues Unt Digital Library

Card News News Notice Information Minisry Of Food And Drug Safety
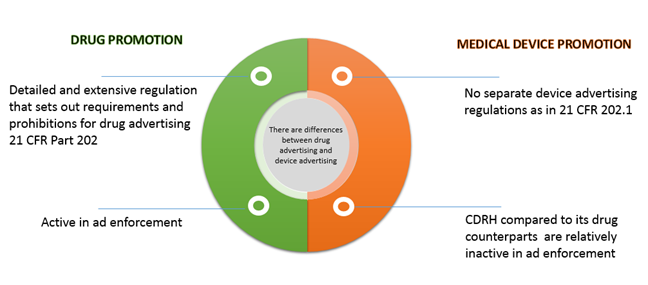
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices

Federal Food Drug And Cosmetics Act By Paulina Castillo

History Of Food Laws And Regulations Ppt Download

Food Drug And Cosmetic Act By Kennedy Smith

Commemorating The 80th Anniversary Of The Passage Of The 1938 Food Drug And Cosmetic Act Food And Drug Law Institute Fdli
Food Additive Reform Time To Repeal The Delaney Clause Food And Drug Law Institute Fdli

Enforcement Of The Food Drug And Cosmetic Act Select Legal Issues Everycrsreport Com

Federal Food Drug And Cosmetic Act 1938 1954 1958 Ffdca Katie Buhai Period Ppt Download

Ppt Federal Food Drug And Cosmetic Act Ffdca Powerpoint Presentation Id 1522252

Legislation Project Federal Food Drug And Cosmetic Act 1938 Catherine Demarco Ppt Download

Legislation Project Federal Food Drug And Cosmetic Act 1938 Catherine Demarco Ppt Download

Federal Food Drug And Cosmetic Act Ffdca By Tara Stull

Post a Comment for "Food Drug And Cosmetic Act Enforcement"