Food And Drugs Act Labelling
No drug shall be considered a generically equivalent drug for the purposes of this chapter if it has been listed by the federal food and drug administration as having proven. Food and Drugs Chap.

Part I The 1906 Food And Drugs Act And Its Enforcement Fda
Food and Drugs Composition and Labelling Amendment.
Food and drugs act labelling. The 1906 Food and Drugs Act. Food labeling is required. The Amendment Regulation was enacted by the.
Through the Food and Drugs Act Health Canada. FOOD AND DRUGS THE FOOD AND DRUGS ACT REGLAONS under section 21 THE FOOD AND DRUGS REGULATIONS 1975 Made by the Minister on the 3rd day of March 1975 LN. Section 206 of the Medical Device User Fee and Modernization Act MDUFMA New section 502 f of the Federal Food Drug and Cosmetic Act Electronic Labeling for.
1040 1938 21 USCA. The Pure Food and Drug Act of 1906 required that specific drugs including cocaine heroin morphine. Answers To Industry Questions About US.
The FDC Act was enacted by Congress to protect consumers from unsafe or deceptively labeled or packaged products by prohibiting the movement in interstate commerce of adulterated or. Subsection 51 of the Food and Drugs Act states. Of the Ghana Standards BoardFood Drugs and other GoodsGeneral Labelling Rules1992LI1541 and is authorised to enter any premises or place to inspect and examine.
301 as amended the drug is required to bear a label containing the legend Caution. The Story of the Laws Behind the Labels By Wallace F. Cosmetic products are subject to the requirements of the Cosmetic Regulationsunder the Food and Drugs Act as well as the Consumer Packaging and Labelling Act.
General Labelling Requirements. These FDA Food Labeling web pages address the labeling requirements for foods under the Federal Food Drug and Cosmetic Act and its amendments. The labelling of drug products is governed by sections 3 9 and 10 of the Act and by sections contained in Parts A C D G and J of the.
The Fair Packaging and Labeling Act FPLA or Act enacted in 1967 directs the Federal Trade Commission and the Food and Drug Administration to issue regulations requiring that all. The United States Federal Food Drug and Cosmetic Act abbreviated as FFDCA FDCA or FDC is a set of laws passed by Congress in 1938 giving authority to the US. The other requirements of this part are issued under both the Fair Packaging and Labeling Act and the Federal Food Drug and Cosmetic Act or by the latter act solely and are.
3001 5 food includes any article manufactured sold or representedfor use as food or drink for man chewing gum and any ingre dient that maybe mixed withfood for. Janssen FDA Historian From FDA Consumer magazine June 1981 Part I. Labelling on food helps Canadians make healthy and informed choices about the foods they buy and eat.
Registrar Corp Reviews Your Labeling Graphics to Assure US FDA Compliance. Labeling Requirements Imposed by the Pure Food and Drug Act. Requirements for Nutrition Labelling and Nutrition Claim Regulation 2008.
No person shall label package treat process sell or advertise any food in a manner that is false misleading or deceptive or is likely to create. Registrar Corp Reviews Your Labeling Graphics to Assure US FDA Compliance. The Federal Food Drug and Cosmetic Act FDC Act and the Fair Packaging and Labeling Act are the Federal laws governing food products under FDAs jurisdiction.
Helping you make healthy choices. Answers To Industry Questions About US. A Under the Food Drug and Cosmetic Act 52 Stat.

Pure Food And Drug Act Facts Worksheets For Kids

Pure Food And Drug Act Facts Worksheets For Kids

Pure Food And Drug Act Facts Worksheets For Kids

What Information Must Your Food Label Contain Jet Label
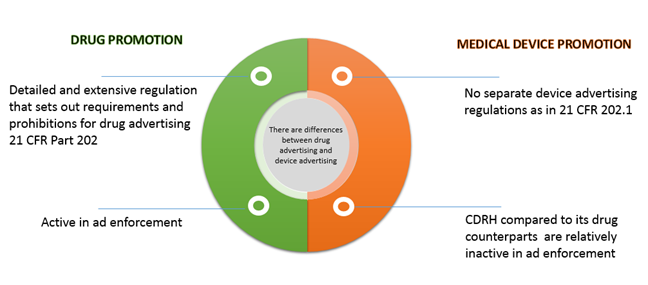
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
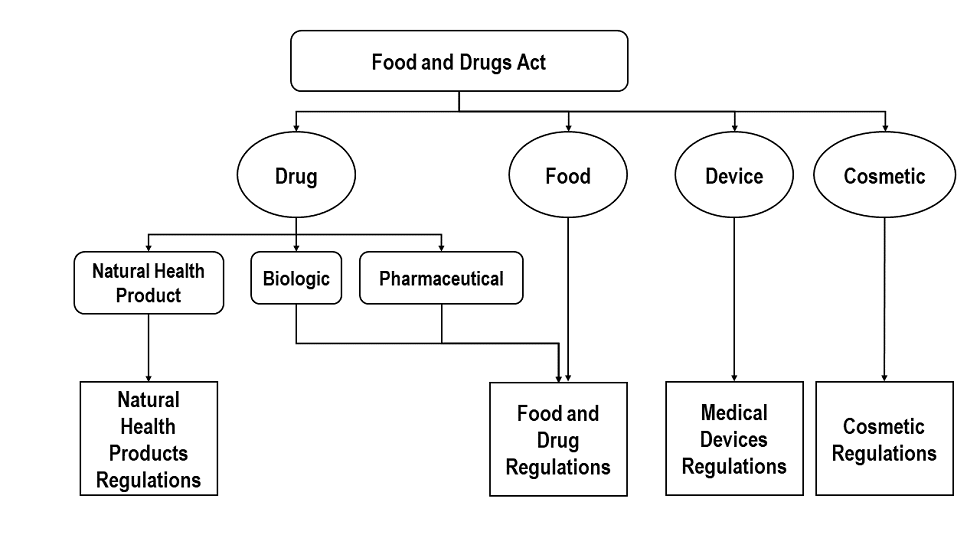
Classification Of Products Under The Food And Drugs Act F Da Canada Ca

Pure Food And Drug Act Facts Worksheets For Kids
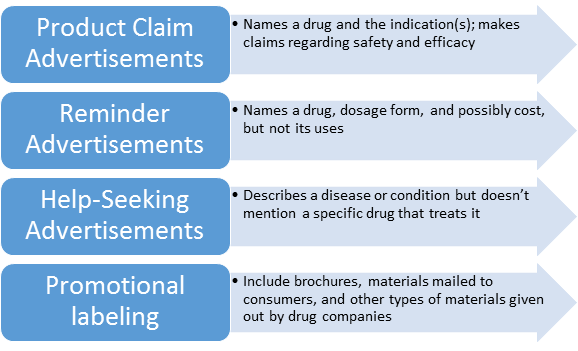
Understanding The Fda Regulations Governing Advertising And Promotion Of Drugs And Medical Devices
Fda Drug Labeling And Ingredient Requirement Fda Registration Assistance

Departmental Consolidation Of The Food And Drugs Act And Of The Food And Drug Regulations With Amendments To December 19 2001 H41 1 2001e Government Of Canada Publications Canada Ca
.jpg)
Fda Drug Labeling And Ingredient Requirement Fda Registration Assistance

Usda Ers Do Food Labels Make A Difference Sometimes

Pure Food And Drug Act Facts Worksheets For Kids

Part I The 1906 Food And Drugs Act And Its Enforcement Fda

Pure Food And Drug Act An Overview Sciencedirect Topics

Food Business Boot Camp Webinar Series Presented By

Food Labeling Legislation What Information Do Food Labels Provide Consumers With Why Are Food Labels Regulated By The Government Ppt Download
Habitina An Infallible Cure For Addiction
Post a Comment for "Food And Drugs Act Labelling"